Semmelweis University
Department of Medical Biochemistry
Department of Medical Biochemistry


SOLID TUMORS OR HEALTHY TISSUE FOR RPPA
Ship tumors/tissues (min 20 mg, max 200 mg) in the designated cryovials following the instructions shown in 'sample submission' link, in dry ice (minimum 5 kg box). Please do not overfill the cryovial with tumor or tissue, see image below.
Ship tumors/tissues (min 20 mg, max 200 mg) in the designated cryovials following the instructions shown in 'sample submission' link, in dry ice (minimum 5 kg box). Please do not overfill the cryovial with tumor or tissue, see image below.

Protocols
CELL PELLET PREPARATION FOR RPPA
Attached cells:
1. Collect a minimum of 2 million cells by either Trypsin EDTA or scraping
2. Wash cell pellet twice with PBS (re-suspend and centrifuge, 2.5 min @3,500 rpm)
3. Aspirate PBS as much as possible without disturbing cell pellet
4. Store dry cell pellet in -80 oC
5. Ship samples in dry ice (minimum 5 kg box)
Suspension cells:
1. Collect a minimum of 2 million cells by centrifugation (2.5 min @3,500 rpm)
2. Wash cell pellet twice with PBS (re-suspend and centrifuge)
3. Aspirate PBS as much as possible without disturbing cell pellet
4. Store dry cell pellet in -80 oC
5. Ship samples in dry ice (minimum 5 kg box)
Along with the cell pellets please send us the information requested as detailed in 'sample submission' link in this excel sheet (tab 'Other'). We prefer to obtain this information prior to receiving the samples.
Attached cells:
1. Collect a minimum of 2 million cells by either Trypsin EDTA or scraping
2. Wash cell pellet twice with PBS (re-suspend and centrifuge, 2.5 min @3,500 rpm)
3. Aspirate PBS as much as possible without disturbing cell pellet
4. Store dry cell pellet in -80 oC
5. Ship samples in dry ice (minimum 5 kg box)
Suspension cells:
1. Collect a minimum of 2 million cells by centrifugation (2.5 min @3,500 rpm)
2. Wash cell pellet twice with PBS (re-suspend and centrifuge)
3. Aspirate PBS as much as possible without disturbing cell pellet
4. Store dry cell pellet in -80 oC
5. Ship samples in dry ice (minimum 5 kg box)
Along with the cell pellets please send us the information requested as detailed in 'sample submission' link in this excel sheet (tab 'Other'). We prefer to obtain this information prior to receiving the samples.
Along with the tumor/tissues please send us the information requested as detailed in 'sample submission' link in this excel sheet (tab 'Living human subject' or 'post-mortem', depending on the origin of the sample). We prefer to obtain this information prior to receiving the samples.

The protocols appearing below are performed by our RPPA team:
IMMUNOSTAINING RPPA USING DAKO AUTOSTAINER
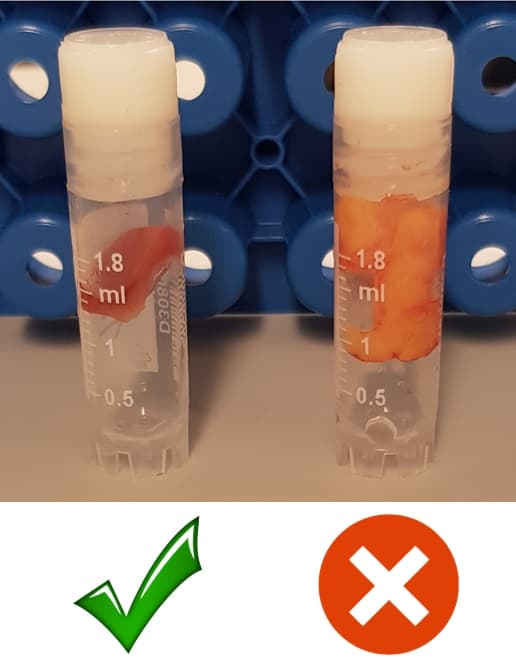
Include only one tube per tumor or healthy tissue or stroma. Do not submit multiple tubes from the same tumor or healthy tissue or stroma. Also, please try to avoid including blood with the samples, but there is no need to make them completely blood-free.
Backup machine files (epMotion)_Lysates load to two plates_normalizations_buffer transfers_dilutions
Slide laser scanning using Innoscan 710-IR